The Biological Research Unit (BRU)
The Biological Research Unit (BRU) at Racing Analytical Services Ltd is a specialised analytical laboratory testing for performance enhancing proteins and peptides. The laboratory was established in 2012 with a grant from the State Government of Victoria to develop assays for protein, peptide and gene doping agents for the Victorian racing industry. The BRU has expertise in the extraction and high-sensitivity detection of bioactive proteins and peptides in urine and plasma. Other research interests include the metabolism and excretion of peptide pharmaceuticals, a field of growing importance as the pharmaceutical industry develops increasing numbers of peptide based drugs.
High-throughput Proteomics.
The Biological Research Unit is a unique research facility, employing experienced biomedical scientists and biochemists within a working high-throughput small molecule drug testing facility. By adopting methods used for small-molecule drug analysis, the laboratory is developing high-throughput proteomic methods with acquisition times as low as 5 minutes per analysis. High-throughput sample preparation based on solid-phase extraction provides efficient sample preparation for high sample volumes. Sample processing efficiency will be improved further by automation with liquid handling robots. The BRU also has interests in developing multiplexed immunoassays for the efficient screening of proteins in plasma.
Using Science to Protect the Integrity of Sport.
Since the Biological Research Unit began operating at the beginning of 2012, the laboratory has been able to respond rapidly to the Dermorphin (peptide opiate) doping scandal that occurred in the USA in late 2012 and to the alleged use of peptides by Ausralian footballers in 2013. The laboratory has developed a unique high-throughput peptide screen for 27 different peptides in urine and plasma based on mixed mode solid phase extractions, and incorporating on column peptide derivatisation for enhanced chromatography. Using this assay, the BRU is able to economically detect peptides in urine and plasma with rapid LC/MS analysis. The laboratory also operates a high-throughput LC/MS based assay for IGF-1, a biomarker for growth hormone use.
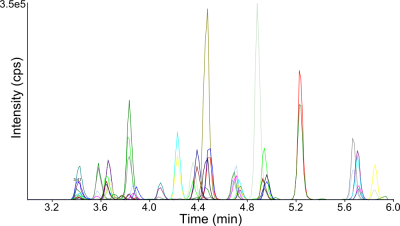
High-throughput screening for 18 dermorphins, 8 GHRP and TB-500 in equine urine.
Policing the inappropriate use of technology
It is now commonplace to hear about the latest scientific discoveries in the media. Modern biology is a productive field of science which is constantly producing new ideas, new pharmaceuticals and new opportunities. Most academic and industrial researchers are focussed on producing positive outcomes from their research and development projects. However, these same technologies can be exploited for other, less desirable applications. This is especially true when the technical developments outstrip the regulatory frameworks, or even the technical capacity for regulation. The BRU is tasked with tackling this technical arms war, exploring the ways modern biomedical science can be applied by people operating in black and grey markets. Protein and peptide pharmaceuticals are increasingly sold in an unregulated market to athletes, sporting clubs and for geriatric medicine. A good example of this industry is the alleged use of the peptides by the Essendon football club and the science graduates who facilitated this process without ethical or scientific governance. Government and industry regulators are required to protect the consumers who are purchasing these products, both for the benefit of the public but also to protect the reputation of the biomedical and pharmaceutical industries.
Publications:
- A high throughput screen for 17 Dermorphin peptides in equine and human urine and equine plasma.Steel R, Timms M, Levina V, Vine J.Drug Test Anal. 2014 Sep;6(9):909-21. doi: 10.1002/dta.1585. Epub 2013 Nov 20.
- A high-throughput LC-MS/MS screen for GHRP in equine and human urine, featuring peptide derivatization for improved chromatography.Timms M, Hall N, Levina V, Vine J, Steel R.Drug Test Anal. 2014 Feb 24. doi: 10.1002/dta.1624. [Epub ahead of print}]